Stimulated by Yan et al 2024.[1]
PD – Parkinson’s disease
IF – impact factor
RCT – randomised controlled trial
PDSS – Parkinson’s disease sleep scale
UPDRS-III – unified Parkinson’s disease rating scale part 3 (motor examination)
MCID – minimum clinical important difference– key to acronyms
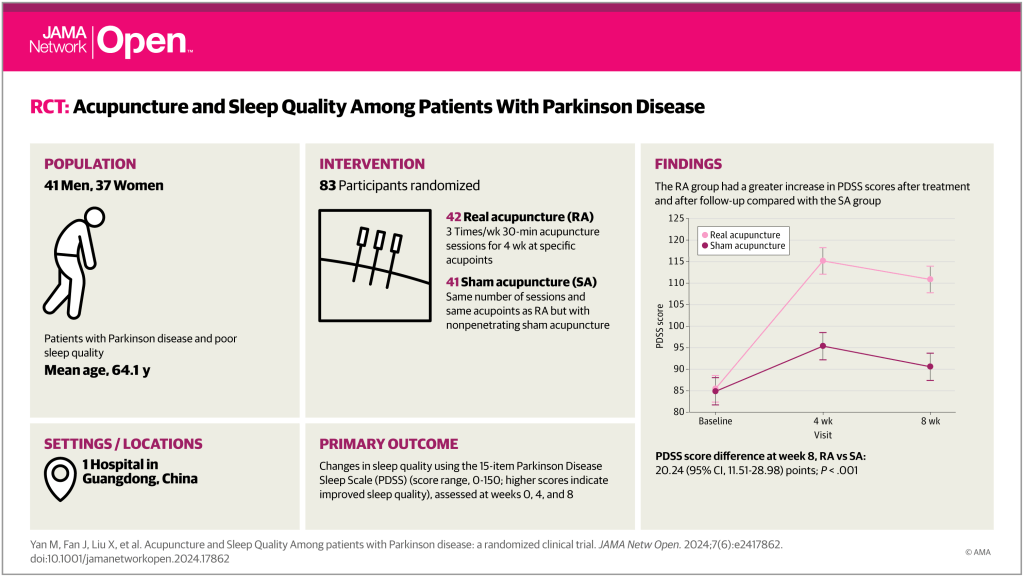
This paper in JAMA Network Open (IF 10.5) reports on a single-centre sham controlled RCT (n=83) from Guangzhou.
The paper comes from the same group that published a similar study on perimenopausal GAD last year (see: Acupuncture for perimenopausal GAD). They used a novel sham acupuncture device that allowed both perpendicular and oblique needle insertion in the real acupuncture group and a non-penetrating version at the same points.
From the images of the devices shown in the papers, it seems that they have modified the device to allow multiple angulations of insertion from 90 degrees (perpendicular) to 15 degrees (a shallow oblique angle).
I am not a great fan of these devices because of the way they can impede the real acupuncture (see: Sham devices impede real acupuncture 2023; and: Do sham devices impede real acupuncture?; and: Trust me…); however, if you have to use one, this looks like it could be one of the best.
The protocol involved 6 points on the head (Sishenzhen [4 points around GV20], GV24, Yintang), 4 points on the upper limbs (LI4, HT7), and 10 points on the lower limbs (ST36, SP6, LR3, BL62, KI6). These points were used in both real and sham groups, with the latter having non-penetrating needling. Sessions were 3 times a week for 4 weeks, and outcomes were measured at baseline, 4 weeks and 8 weeks.
The primary outcome was the change in PDSS scores. There were a number of other outcome measures including the UPDRS-III score, which assesses motor signs in PD.
The PDSS assesses 15 items related to sleep and nighttime symptoms, each on 0 to 10 scales, meaning that the total score ranges from 0 to 150, with higher scores indicating better sleep quality.
The baseline PDSS score was 85, and this increased after 4 weeks to 95 in the sham acupuncture group and to 115 in the real acupuncture group. After a further 4 weeks follow-up the scores in both groups dropped back by 5 points to 90 and 110 respectively. A highly significant 20-point difference was maintained between the groups at both time points. It is not easy to judge how important a difference of 20 points is on this scale, and I could not find any mention of MCID for PDSS on PubMed.
I did find an MCID for the PDSS-2 scale,[2] which rates the same questions from 0 to 4, and so has a total score from 0 to 60. The MCID on this scale has been measured as -3.44 points. If we scale this up from 60 to 150, we get an estimate of the MCID for the original PDSS at about -8.6.
So, it does look as though the effect in the real acupuncture was clinically significant. For comparison, I found a study of two different preparations of pramipexole, which measured a 14-point change from baseline on the PDSS-2 scale, and it was in a Chinese population.[3] This would be 35 points on the original PDSS scale. The change from baseline in the real acupuncture group of the current trial was 30, which is not far off, and gives me confidence that there is a real effect from the acupuncture.
I was interested to see a significant benefit of acupuncture over sham on the UPDRS-III (motors signs in PD), and the change from baseline in the acupuncture group just exceeded one estimate of the MCID on this scale.[4]
References
1 Yan M, Fan J, Liu X, et al. Acupuncture and Sleep Quality Among Patients With Parkinson Disease: A Randomized Clinical Trial. JAMA Netw Open. 2024;7:e2417862. doi: 10.1001/jamanetworkopen.2024.17862
2 Horváth K, Aschermann Z, Ács P, et al. Minimal Clinically Important Difference on Parkinson’s Disease Sleep Scale 2nd Version. Park Dis. 2015;2015:970534. doi: 10.1155/2015/970534
3 Zhou H, Li S, Yu H, et al. Efficacy and Safety of Pramipexole Sustained Release versus Immediate Release Formulation for Nocturnal Symptoms in Chinese Patients with Advanced Parkinson’s Disease: A Pilot Study. Park Dis. 2021;2021:8834950. doi: 10.1155/2021/8834950
4 Hauser RA, Gordon MF, Mizuno Y, et al. Minimal clinically important difference in Parkinson’s disease as assessed in pivotal trials of pramipexole extended release. Park Dis. 2014;2014:467131. doi: 10.1155/2014/467131
You must be logged in to post a comment.