Stimulated by Zhang et al 2025.[1]
EA – electroacupuncture
MIA – maternal immune activation
IF – impact factor
APC – author processing charge
USD – US dollars
Poly (I:C) – polyinosinic-polycytidylic acid
RNA – ribose nucleic acid
IL6 – interleukin 6 (a proinflammatory cytokine)
MCP-1 – monocyte chemoattractant protein 1 (a downstream chemokine of IL6)
α7nAChR – the α7 acetylcholine receptor (located on splenic macrophages and elsewhere)
NTS – nucleus tractus solitarius
PVN – paraventricular nucleus
AAV – adeno-associated virus
DREADD – designer receptor exclusively activated by designer drug– key to acronyms
This paper in the journal Cell Reports (IF 6.9 – but was higher) comes from Jinan in China. Jinan is the capital of Shandong province and lies roughly one third of the way from Beijing to Shanghai. Shandong province is on China’s eastern coastline and includes a large peninsula jutting into the Yellow Sea and pointing towards Seoul at the top left of South Korea.
This is the first paper published in Cell Reports with EA in the title. There are no papers with acupuncture in the title. The last author (the boss and corresponding author) has never published in this journal before, although he has published in several other moderately high impact journals. Cell Reports was established in 2012 as the first open access journal from Cell Press, and it has an eye-watering APC (USD 5 620).
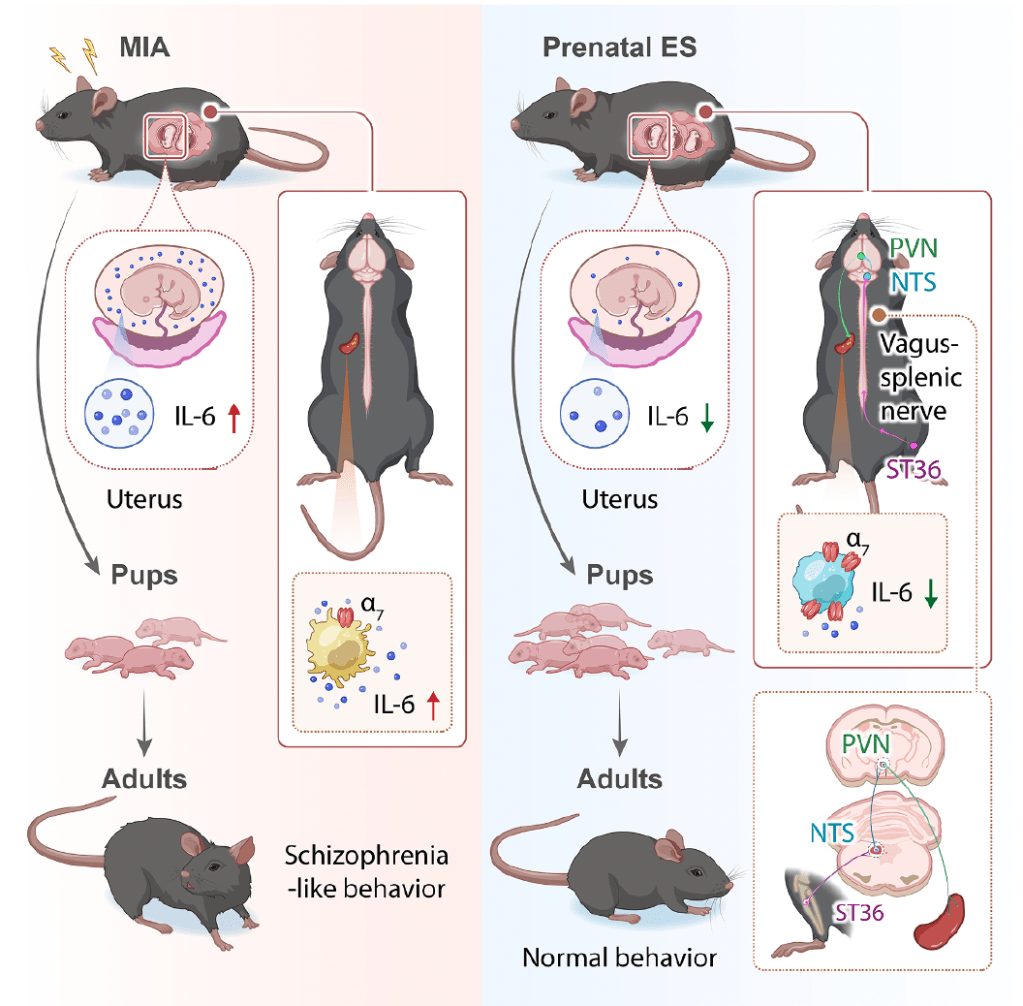
This is one of those laboratory papers with numerous different experiments within it that builds up an almost complete picture of the mechanism of pre-natal EA in MIA. It starts by establishing the model and demonstrating that EA can reverse the important outcomes, which are fetal survival and schizophrenia-like behaviours in MIA offspring.
The MIA model was created by intravenous injection of poly (I:C) into pregnant C57BL/6 mice. Poly (I:C) is a synthetic double-stranded RNA, which is used to mimic viral infection. It generates immune activation in a similar manner to influenza, Zika, or COVID-19.
EA through pairs of needles at ST36 bilaterally was applied daily for 6 days from the day following the injection of poly (I:C). EA was applied for 15 minutes at 10Hz and a pulse width of 50μs using a rather expensive EA device (AMPI Master 8). This is not really an EA device, but a pulse generator designed for neuroscience experiments. Initially, 3 different intensities were tried – 0.1mA, 0.5mA, and 1.0mA. The optimal strength turned out to be 0.5mA, so this was adopted for the remainder of the experiments.
Of 12 different cytokines and chemokines studied, only 2 turned out to be substantially (>5-fold) increased in maternal serum – IL6 and MCP-1. These were also significantly increased in fetal brains.
Anti-IL6 or anti-MCP-1 applied at the time of modelling had the same effect as a course of EA.
The MIA model resulted in a marked (2-fold) increase in splenic macrophages but little or no effect on T lymphocytes. M1 (proinflammatory) macrophages increased, while M2 (anti-inflammatory) macrophages decreased. These changes were reversed by EA and anti-IL6.
Next the team turned their attention to the role of the α7nAChR, which was first made famous when the vagal anti-inflammatory reflex was described.[2] First they established that splenic acetylcholine was affected in the model but dopamine was not and that dopamine receptor 1 knockout mice responded to EA in the same way as model mice. In α7nAChR knockout mice, the effect of EA was lost. To be certain though, they also blocked the receptor the with α-bungarotoxin, and even used a genetic manipulation to disable just the a7 receptor on macrophages to determine that this receptor on splenic macrophages was critical to the protective effect of EA.
The final steps involved interrogating the neural control of this anti-inflammatory pathway. Cutting the vagus in the neck blocked the effect of EA, as did artificially reducing neural activity in both the NTS and PVN. Sounds simple, but that last bit involved a number of steps including fluorogold, c-fos, stereotactic injection of AAVs, and a DREADD or two.
So, we have another sophisticated experimental paper demonstrating the mechanism of action of one of my favourite treatment approaches, but this time in a completely novel clinical situation. I wonder how long it might take before we see it in practice. Perhaps our very own maternofetal medicine expert can hazard a guess on Wednesday at the webinar.
References
1 Zhang Z, Lin W, Yan J, et al. Prenatal electroacupuncture modulates maternal-fetal immune activation via a brain-to-splenic signal. Cell Rep. 2025;44:116576. doi: 10.1016/j.celrep.2025.116576
2 Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321
You must be logged in to post a comment.