Stimulated by Yu et al 2025.[1]
EA – electroacupuncture
CACMS – China Academy of Chinese Medical Sciences
IF – impact factor
JIM – Journal of Integrated Medicine
JCIM – Journal of Chinese Integrated Medicine
CJIM – Chinese Journal of Integrated Medicine
CFA – complete Freund’s adjuvant (an oily antigenic mixture including heat-killed mycobacteria)
SD – Sprague-Dawley
WDR – wide dynamic range (neurons in lamina 5 of the SDH responsive to both noxious and non-noxious stimuli)
SDH – spinal dorsal horn
RF – receptive field (in this case referring to the WDR neurons being monitored)
LTP – long term potentiation (refers to synaptic transmission in C fibres here)
MSK – musculoskeletal– key to acronyms
I am pleased to be able to reclaim the EA acronym this week by highlighting a paper from Professor Jing’s laboratory at CACMS in Beijing. It is published in the Journal of Integrated Medicine or JIM (IF 4.0).
JIM was originally JCIM, not to be confused with other similar journal acronyms where the C stands for Complementary rather than Chinese (see EA for hot flushes 2025). JCIM was established in 2003 but was published in Chinese until 2013. It is based in Shanghai. Note that there is also a CJIM, which dates back to 1995 under a longer name.
The paper was published online right at the end of December, so it has 2025 in its citation as I write this, but it will inevitably change to 2026 when it reaches an issue of the journal.
This is an experimental study using CFA to create a model of inflammatory muscle pain in the left gastrocnemius muscle of SD rats. EA was used at different intensities in an attempt to mitigate the mechanical allodynia (hence muscle tenderness above) and related neural activation in the ipsilateral spinal cord. The latter was performed in vivo using rather sophisticated microelectrodes.
The first time we saw such electrodes used was in a paper from the same group that I highlighted in March 2023 (see Pre-EA on spinal WDRs).[2] In this paper from 2 years ago, EA was used prior to a noxious injection (hypertonic saline) in the left gastrocnemius muscle of SD rats and output from WDR neurons in the ipsilateral SDH was monitored. This WDR output, which would theoretically correlate the intensity of perceived pain from the stimulus, was reduced by high intensity (Aδ or C) pre-EA in the same receptive field (RF). Only the highest intensities (C or more) worked if the pre-EA was applied to the opposite leg, but interestingly, low intensity (Aβ) did work when applied to an adjacent ipsilateral RF.
So, the current experiment is rather similar, but the model is a bit more like what we would see in clinical practice, ie the pain already exists and we try to reduce its impact on function and symptoms.
CFA injection creates persistent inflammation and so represents something different from an acute traumatic muscle injury and more like an immune-driven pathology lasting for weeks after a single injection. Whilst the pathology is not the same as we see in the clinic, the persistent mechanical allodynia created in muscle tissue may be similar in terms of the peripheral and central neural sensitivity that is set up.
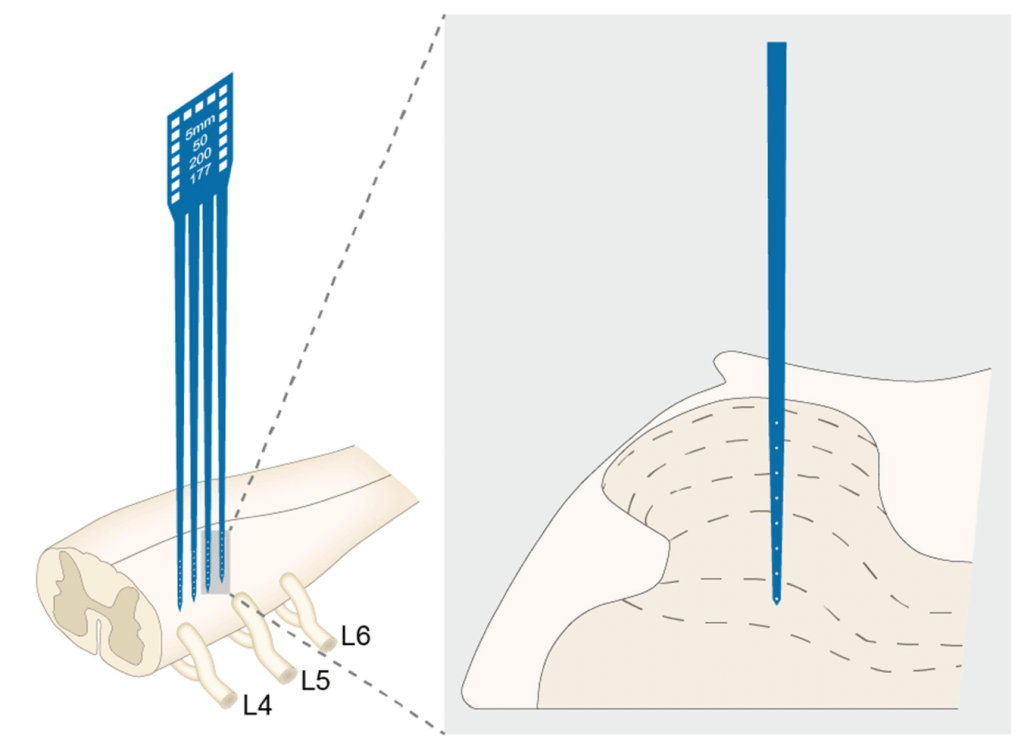
Mechanical allodynia was measured in the usual ways, but the most interesting outcomes were the direct measurement of neural activity in the SDH on day 5 of the study. These involved recording from WDR neurons in the deep SDH (microelectrodes inserted 0.6 to 1.4mm) and from C fibres in the more superficial SDH (microelectrodes inserted 0.3 to 0.5mm). Electrical test stimuli were applied to the plantar surface of the ipsilateral hind paw and both the immediate and delayed (post-stimulation to assess wind-up) response of WDR neurones was recorded. To measure LTP in the superficial SDH, the recordings were continued for 3 hours following the test stimuli.
EA was applied on days 3, 4, and 5, and the CFA injection was applied on day 0. EA was applied to the ipsilateral ST36 using a pair of needles (0.18 x 13mm) inserted 5mm. The frequency used was 2/15Hz alternating (pulse width of 500μs) and the intensity was set at 0.5, 1.0, or 2.0mA. The latter intensities were known to stimulate, Aδ, and C fibres respectively.
The model demonstrated persistent allodynia from day 1 to day 5. This was diminished from day 3 to day 5 by the higher intensity EA (Aδ and C), but not by the lower intensity (Aβ). The higher intensity EA reduced WDR discharge, but only the C fibre level (2.0mA) reduced wind-up in WDR neurones and LTP in the superficial SDH.
As the authors note, there is still much work to be done to elucidate the molecular mechanisms involved in these effects; however, this rather sophisticated in vivo monitoring of spinal nerves gets us a step closer to seeing what is happening in our patients in the clinical setting when they have immediate reduction in pain symptoms for chronic peripherally-driven MSK pain.
References
1 Yu Q-Q, Sun X-Y, Chen J-K, et al. Distinct intensity of electroacupuncture ameliorates mechanical hypersensitivity by attenuating neuronal sensitization in spinal dorsal horn in a rat model of inflammatory muscle pain. J Integr Med. 2025;S2095-4964(25)00202-X. doi: 10.1016/j.joim.2025.12.010
2 Yu Q, Cao W, Wang X, et al. The Effect of Pre-Electroacupuncture on Nociceptive Discharges of Spinal Wide Dynamic Range Neurons in Rat. J Pain Res. 2023;16:695–706. doi: 10.2147/JPR.S396481
You must be logged in to post a comment.